8.9 billion! The money that these big guys are doing research and development burns almost half of the industry!
As of press time, a total of 172 pharmaceutical companies in the A-shares disclosed the 2017 annual report. In terms of R&D investment, the research and development investment of 137 pharmaceutical companies that published data totaled 19.7 billion yuan, and the innovative pharmaceutical company was the most “squatting†camp for R&D investment in pharmaceutical companies. Image source: Petal beauty The total R&D investment of 18 innovative pharmaceutical companies that have disclosed the 2017 annual report is 8.886 billion yuan, accounting for 45% of the total R&D investment of pharmaceutical companies. Among them, Hengrui Medicine 600276, Share Bar and Fosun Pharma 600196, the R&D investment of the stock bar exceeds It is 1.5 billion yuan. As we all know, the innovation of the pharmaceutical industry has the characteristics of large investment, long-term and high-risk. It takes more than 10 years for products to go from research and development to listing. Under the investment of innovative pharmaceutical companies, what are the expectations of each company in 2018? Pound products? What is the investment logic of the innovative medicine industry? R&D investment: the arena of leading companies Wind data shows that 18 innovative pharmaceutical companies have released the 2017 annual report, with a total investment of 8.886 billion yuan, with an average investment of nearly 500 million yuan per research and development. The R&D investment of more than 1 billion yuan is Hengrui Medicine and Fosun Pharma, which are 1.759 billion yuan and 1.529 billion yuan respectively, leading all other pharmaceutical companies; Haizheng Pharmaceutical Co., Ltd. 600267, R&D investment of more than 500 million yuan Medicine 601607, stock bar, Renfu Medicine 600079, stock bar and Livzon Group 000,013, stock. In fact, research and development has always been only a leading enterprise arena. Only leading pharmaceutical companies with scale advantages and capital advantages can support the huge R&D system costs, form stable sales cash flow with heavy varieties, and then provide stable support for R&D investment. More innovative varieties after the development cycle can be put into the market. A number of innovative pharmaceutical companies have indicated that they will make more progress in research and development. Hengrui Pharmaceutical's R&D investment in 2017 increased by 48.53% year-on-year, and R&D investment accounted for 12.71% of sales revenue. The company insisted on investing R&D funds of about 10% of annual sales. Hengrui Medicine said that in 2018, the company will further increase investment in research and development, focusing on anti-cancer drugs, surgical drugs, cardiovascular and cerebrovascular, contrast agents and biomedicine. Fosun Pharma proposed that the research and development expenses of the pharmaceutical business in 2018 should not be less than 5% of the sales revenue of the pharmaceutical business. In 2017, Fosun's R&D investment increased by 38.26% year-on-year, accounting for 8.3% of operating income. R&D investment increased by 74.84% year-on-year. Huadong Pharmaceuticals expects that the expenditure on R&D expenses in 2018 will increase by more than 30% in 2017. The company will accelerate the progress of new product research and development, new product development costs and technology introduction. The investment will be further increased. R&D motivation: good times and people Why are innovative drug companies so willing to do so in research and development? One of the most important factors is the rapid growth of the market, and the second is the support of the medical policy and the drug trial environment. According to the latest information from the Consumer Products Industry Department of the Ministry of Industry and Information Technology, from January to September 2017, the pharmaceutical industry's above-scale enterprises achieved a main business income of 2,293,645 million yuan, a year-on-year increase of 11.70%, an increase of 1.61 percentage points over the same period of the previous year; 255.726 billion yuan, an increase of 17.54% over the same period of last year, an increase of 1.9 percentage points over the same period of the previous year. Among the sub-sectors, the fastest growth in profits was in biopharmaceutical manufacturing and chemical formulation manufacturing, which increased by 26.26% and 24.79% respectively. Traditional chemical applications, such as chemical synthesis or biosynthesis, are small molecule drugs that use natural minerals or active ingredients extracted from plants and animals to perform certain structural modifications as needed. However, from a technical perspective, in the domestic chemical pharmaceutical preparation industry, generic drugs still account for the vast majority, and the types and quantities of drugs independently developed are seriously insufficient. At present, China's chemical pharmaceutical preparation industry has entered a stage of rapid differentiation, structural upgrading, and elimination of backward production capacity. Enterprises with independent medical innovation capabilities and intellectual property protection will be in a dominant position in the future chemical preparation competition market. Biopharmaceuticals are products for the prevention, treatment and diagnosis that are manufactured by the principles and methods of microbiology, chemistry, biochemistry, biotechnology, pharmacy, etc. It is a macromolecular drug with a structure than a chemical. More complex, more difficult to develop and produce. Generalized biological drugs include enzymes, cytokines, rapids, antibodies, vaccines, blood products, gene therapy drugs, cell therapy drugs and other major categories. In recent years, with the biotechnology revolution, the research and development of various biomacromolecules, especially antibody drugs, has become a hot spot in the development of new drugs worldwide. Evaluatepharma data shows that antibody drugs currently account for about 40% of the total biotech drug market, and market share continues to grow. It is estimated that by 2022, global antibody drug sales will reach 170.8 billion US dollars, and the proportion of biotech drugs will be Rose to 52%. The development of biopharmaceuticals, especially antibody drugs, is still in its infancy. At present, quite a few biomacromolecules approved in the European and American markets have not been listed in China. The development of biomacromolecules in China is still in the stage of learning, introduction and imitation. . Because the imitation of biopharmaceuticals is also very difficult, the imitation process is almost equivalent to a re-development. If the company can enter the market through R&D, its importance is self-evident. There is also a medical policy that can not be ignored. In 2017, the pharmaceutical industry ushered in major changes. The government departments at all levels of the country have issued more than 1,700 pharmaceutical industry policies, including more than 300 national-level policies, covering various aspects such as medicine, medical care, and medical insurance resources. Among them, in terms of medical policy, the first is to encourage innovation, from the protection of new drug patents, the expansion of clinical institutions, the system of drug listing license holders, and the speeding up of examination and approval, etc., to guide the establishment of a complete R&D and listing path and promote industrial restructuring. And technological innovation; The second is to improve product quality. The State Food and Drug Administration (CFDA) has become a member of the International Technical Committee for the Registration of Pharmaceuticals for Human Use. This means that China's drug regulatory authorities, pharmaceutical industry and research and development institutions will gradually implement the highest international technical standards and guidelines. In another example, on October 8, 2017, the CFDA issued the “Opinions on Deepening the Reform of the Examination and Approval System to Encourage Innovation in Pharmaceutical Medical Devices†and vigorously supported the development of innovative drugs in China. On December 28, 2017, the CFDA again supplemented the comments on encouraging priority review and approval of drug innovation, and continuously promoted the development of innovative drugs. Hengrui Medicine believes that with the tightening of drug registration conditions, the strengthening of new drug approval supervision and the promotion of generic drug quality consistency evaluation, China's pharmaceutical industry will gradually achieve the survival of the fittest, and it is expected that a group of enterprises with advantages in capital and research and development will take advantage of the situation. Complete scale expansion and invest more resources in innovative R&D. Beida Pharmaceutical believes that the development of innovative drugs in China has the advantages of time, location and people. In the next few years, innovation will be the main theme of the development of the pharmaceutical industry. Anti-tumor targeted drugs will also usher in a climax and firmly grasp the anti-tumor. The main line of drug development will be an important key for domestic pharmaceutical companies to achieve overtaking in corners. R & D speed: how attractive is the monopoly The malignant disease represented by tumor is the main indication of biopharmaceuticals, and the antibody drug is the "top card" in biopharmaceuticals. As in the case of Beida Pharmaceutical, anti-tumor drugs play an important role in this development cycle of innovative drugs. It is understood that 8 of the top ten global sales in 2017 are biopharmaceuticals, 7 of which are monoclonal antibodies (ie, monoclonal antibodies, the most important of the antibody classes), and sales of monoclonal antibodies account for 73.5% of the top ten drugs. Southwest Securities 600369, shares it believes that biopharmaceuticals is the most valuable pharmaceutical market segment in the next 10 years. According to Frost & Sullivan, the market size of biopharmaceuticals in China increased from 62.7 billion yuan in 2012 to 152.7 billion yuan in 2016, with a compound annual growth rate of 24.9%. It is expected to grow at a compound annual growth rate of 16.4% from 2016 to 2021 and reach a market scale of 326.9 billion yuan in 2021, which will bring huge opportunities for Chinese biopharmaceutical participants. Of the 44 drugs selected for inclusion in the China National Health Insurance Negotiation Catalogue in 2017, 14 were biopharmaceuticals, accounting for about 32%, of which 8 were antibody-based drugs, accounting for 57% of the selected biopharmaceuticals. It can be seen from the international and domestic environment why domestic innovative pharmaceutical companies use such strength to engage in research and development. On the other hand, the competitiveness of biopharmaceutical R&D pharmaceutical companies is determined by the time to market, the number of competitors and the market space, so the importance of R&D to market speed is self-evident. Taking Hengrui Medicine as an example, its three major businesses in the anti-cancer and anesthetic business grew steadily, and the contrast agent maintained a high-speed growth. The anti-tumor sales revenue was 5.72 billion yuan, a year-on-year increase of 18.5%. In 2017, Hengrui Medicine listed two innovative drugs, ericoxib and apatinib, among which apatinib is suitable for advanced gastric adenocarcinoma or gastric-esophageal junction adenocarcinoma. Still maintaining market leadership, 36 types of negotiating drugs such as apatinib were included in the 2017 edition of the National Health Insurance Catalogue. In 2017, Hengrui Medicine has 17 innovative drugs in clinical development. In the development of innovative drugs, the company has basically formed a clinical application for innovative drugs every year, and the development trend of innovative drugs is listed every 2-3 years. In 2017, it is an important year for Hengrui Medicine in the field of innovative drug research and development. 19K, pyrrolidine and paclitaxel (albumin combined) have completed production declarations. Among them, pyrrolidine treatment for breast cancer treatment completed Phase II clinical trial in December last year, and has been declared for production. It is expected to become the company's first innovative drug variety approved in Phase II clinical data. The antibody drug PD-1 monoclonal antibody targeting the immunodetection site has completed Phase II clinical trial of Hodgkin's lymphoma and is currently in Phase III clinical trial. Fosun Pharma's R&D investment in 2017 is second only to Hengrui Medicine, which has the most potential and growth potential in the domestic pharmaceutical market (cardiovascular, metabolic and digestive systems, central nervous system, blood system, anti-infective, Anti-tumor) has a rich product line. According to the company, the increase in R&D investment in 2017 is due to the continuous increase in concentrated investment in research and development of biosimilar drugs and bio-innovative drugs, small molecule innovative drugs, and consistency evaluation. In 2017, Fosun Pharma strengthened the product layout of anti-tumor drugs. There were 6 monoclonal antibody varieties (including one innovative monoclonal antibody) and 11 indications approved in clinical trials in mainland China. Southwest Securities believes that the monoclonal antibody business in Fosun Pharma will reach 5.028 billion yuan in 2018, which can be said to be the engine of expansion and expansion of Fosun Pharma. Its main product, rituximab (which has been declared for production, is expected to be marketed in 2018 for the treatment of non-Hodgkin's lymphoma), and trastuzumab (preferred to be marketed in 2019) is estimated to bring cash flow. The value is 3.5 billion yuan and 3 billion yuan. In 2017, Hisun Pharmaceuticals invested more than 800 million yuan in research and development, and plans to launch 20 important research and development projects in the new year, 8 of which are chemical drugs 1.1 (ie, innovative drugs not listed at home and abroad). Beida Pharmaceuticals, which focuses on the treatment of malignant tumors, diabetes, cardiovascular diseases, etc., currently has more than 30 projects under development, of which 7 have entered the clinical trial phase. Watson Bio 300142, a combination of vaccine and monoclonal antibody, representative products include 23-valent, 13-valent pneumonia vaccine and HPV vaccine and bio-pharmaceuticals, represented by subsidiary Jiahe Bio, leading in the research and development of monoclonal antibody, the company has 10 The remaining products are in various stages of development. Investment logic Overall, the pharmaceutical industry has been a major investment hot spot since the first quarter of this year. Wind data shows that since the concept of innovative medicines was included in the index, its increase has been leading the Shanghai Composite Index since the third quarter of last year. After a period of correction at the beginning of the year, the sector index has been exerted again since February this year. Southwest Securities forecasts that Zhifei Bio is rich in research and variety, and the company's follow-up product echelon reserves are abundant. The company's self-developed freeze-dried Hib vaccine, 15-valent pneumonia vaccine and freeze-dried human rabies vaccine (MRC-5) have successively obtained clinical approvals, predicting that the company's performance will show an explosive growth in the next three years. It believes that Hengrui Pharmaceutical will enter the period of innovative drug outbreak from 2019 to 2021. It is expected that the three-year compound growth rate of revenue and performance will reach 30% and 37% respectively, and the value of the company's existing and research product lines and R&D platforms will be separately carried out. Valuation, the company's reasonable relative market value in 2018 is about 265 billion. Zhongtai Securities Research believes that Shuanglu Pharmaceutical's research varieties cover a number of advantageous areas. In addition to strengthening its advantages in the fields of cancer, liver disease, metabolic diseases, cardiovascular and cerebrovascular diseases, the company has gradually entered new diseases such as diabetes, kidney disease and preventive vaccine. field. In 2018, with the launch of the heavy variety lenalidomide, the company's performance turning point is expected to appear in 2018. The main focus is on two aspects: First, the ability to sell products is profitable. Only by maintaining stable and long-term profitability can we maintain long-term, high-intensity capital investment in innovative drug research and development. Second, the first-class drug research and development team, with more research and development accumulation, has developed a complete and rich product research echelon is very important. This article was first published on WeChat public account: e company official micro. The content of the article belongs to the author's personal opinion and does not represent the position of Hexun.com. Investors should act accordingly, at their own risk. (Editor: He Yihua HN110) Baby Shoes,Oft Baby Sandals,Slip On Casual Shoes,Comfortable Shoes For Baby Huaian sunrise shoes Co.,Ltd , https://www.jssunriseshoes.com
Among the 18 pharmaceutical companies, in addition to the year-on-year decline in Liaoning R&D investment, the R&D investment of other innovative pharmaceutical companies increased to different degrees year-on-year. The research and development investment of Beida Pharmaceuticals increased the most, nearly 136%. Other companies including Huadong Pharmaceutical The R&D investment of Anke Bio, Yifan Bio, Hengrui Medicine, Xinlitai, Fosun Pharma and Hualan Biotech both increased by more than 30% year-on-year. 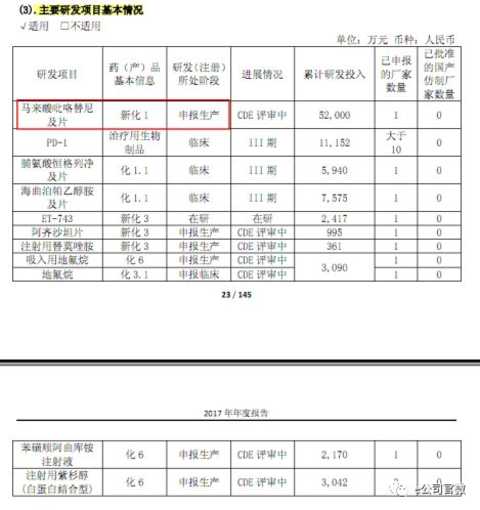
Southwest Securities believes that after 2017, Hengrui Pharmaceutical's R&D investment is expected to achieve comprehensive harvest in 2018. It is expected that there will be 19K, paclitaxel (albumin-binding), pyrrolidine, PD-1, remazolam and so on. The pound variety was approved for listing. 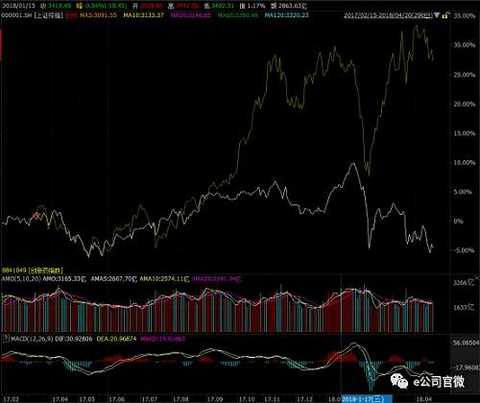
From the stocks, since January this year, the most leading stock price increase is Kangtai Biological (36.11%), followed by Shuanglu Pharmaceutical (28.77%), Zhifei Biological (28.68%), Huadong Medicine (25.56%) and Hengrui Medicine (21.82%). 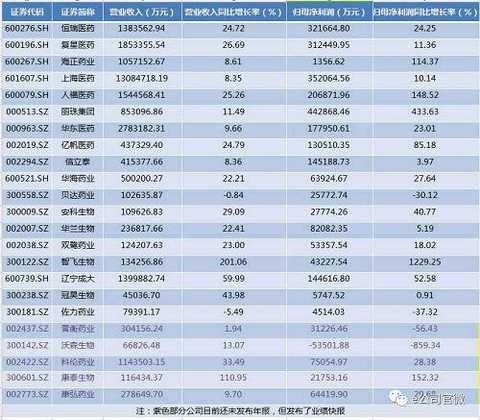
A private equity researcher in Shenzhen who specializes in investing in the pharmaceutical industry said that innovative drugs have a high degree of uncertainty in input and output, coupled with high intensity and long-term investment, preferring to choose large pharmaceutical companies.